A discussion of gland mechanisms, how they can go awry and what you can do to nurse them back to health.
Meibomian glands are essential pieces of the homeostatic machinery that keep the ocular surface clean, healthy and well-lubricated. Dysfunction of these glands is a common disorder, with a widespread prevalence of 39 to 50 percent of the population that increases with age.1,2 It’s characterized by alterations in gland morphology and location, as well as a waning in quality and quantity of gland secretion. The etiology of meibomian gland dysfunction is unknown and may be due to any one of a variety of conditions, including bacterial infection, hormonal imbalance, autoimmune disease, inflammation, conditions inducing premature cell death or external mechanical factors. This month’s column, the second half of our discussion of eyelid health, focuses on the anatomy and physiology of sebaceous and meibomian glands, the pathophysiology and classification of MGD, diagnostic tools and current and emerging treatment options.
The Anatomy
Both upper and lower eyelashes are coupled to two types of secretory glands: the sebaceous glands of Zeis and the apocrine (sweat) glands of Moll. Secretions from these glands protect the eyelid surface, while the glands of Meibum, positioned between the lashes and the bulbar conjunctiva, exude a fat and oil mixture onto the ocular surface. There are approximately 20 to 30 meibomian glands on the lower lid and 40 to 50 on the upper lid. Healthy glands are easily visualized by illumination of either lid, and appear as grape-like clusters attached to a central stalk. This structure is composed of acinar cells connected to a central duct that opens onto the mucocutaneous junction at the lid margin.3 Lipid secretions containing dozens of oils and waxes are constantly synthesized and secreted, pushing meibum slowly toward the marginal orifice. In addition to this basal exudation, contraction of the Riolan’s muscle coupled with normal blinking causes the meibum to be expressed and spread over the ocular surface. Essential lipids in the tear film reduce evaporation, seal the lid margins during sleep and help maintain the near-perfect optical surface required for visual acuity.3
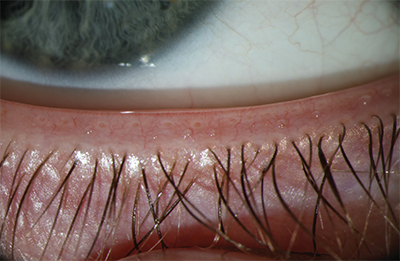 |
| A patient with healthy meibomian gland function. In the normal patient, note how the meibomian gland ducts are visible, clear and properly aligned. |
Pathophysiology
Transillumination of the lid margin reveals anatomical differences between normal and MGD patients. Normal individuals show the grape-like clusters of acini that form long meibomian glands, while MGD patients may have congenitally absent or atrophied glands and gland dropout, as well as enlargement or laxity of the glands, particularly with aging.3 Inflammation may or may not be present and patients may exhibit dry-eye symptoms, chalazia and hordeola.
There is no global consensus on the classification and diagnosis of MGD. The MGD Workshop distinguished meibomian gland disease, a term that encompasses secretory dysfunction as well as congenital or neoplastic conditions, from meibomian gland dysfunction, defined as including only hypo- or hypersecretory conditions.4 For our purposes, we focus on the latter definition, as it includes other proposed categories including hormonal, bacterial and contact lens-associated MGD.5-7Other proposed grading systems, such as anterior and posterior, hypersecretory and hyposecretory, and non-obvious and obstructive provide little practical information on disease etiology and are of little clinical use. When developing a grading system, particularly one designed to assess new therapies, the system should be sensitive and precise enough to detect small but clinically relevant changes in outcome variables.
One of the first steps in translational research is moving from an understanding of the pathophysiology behind diseases such as MGD to a well-constructed clinical trial in which the therapeutic impact of a compound can be evaluated. To this end, researchers at Ora have developed the Lid Margin Disease Digital Image Grading System. This validated, standardized set of scales comprises a structurally based classification system that allows clinicians and researchers to reproducibly measure anatomic and functional components of the lid margin and surrounding structures including the meibomian glands, skin, palpebral conjunctiva, mucocutaneous junction, tear meniscus, lashes and follicles, as well as the degree of inflammation of each. In several clinical trials,8 the grading system has been used to quantify lid margin redness (temporal, medial and nasal regions); palpebral conjunctival redness; lid edge shape; keratinization; lash folliculitis; lash loss; debris; mucocutaneous junction placement; meibomian gland morphology and MG secretion. (Shapiro A, et al. IOVS 2008;49:ARVO E-abstract 84; Blackie CA, et al. IOVS 2008;49:ARVO E-abstract 86) Assessment of secretion includes the number of glands, gland geometry, alignment, height and peri-gland redness, as well as meibum quality, viscosity and color. All assessments were based upon a 0 to 3 visual analog scale. By using scales such as this to assess all aspects of lid health, clinicians can more easily recognize underlying pathophysiology and will be in a position to best know how to treat the underlying dysfunction.
Danish ophthalmologist Mogens Norn was the first to suggest that the Marx line represents the border between the tear film and the skin and corresponds to the mucocutaneous junction.9 As a marker of the tear-film boundary, researcher Masahiko Yamaguchi, MD, and his colleagues at Japan’s Ehime University hypothesized that a more anterior location of the Marx line might be correlated with MGD.10 They found a strong correlation between the position of the line, meibomian gland dropout and abnormal gland secretion.
The Marx line runs parallel to and away from the meibomian orifices along the conjunctival border in normal, younger subjects, while it becomes irregular and moves closer (anteriorly) to the orifices with aging. We don’t yet know whether the development of MGD precedes or is caused by displacement of the Marx line with aging. It seems, though, that meibomian gland dropout and anterior line displacement are related.11
Obstructive MGD might involve hyperkeratinization of the central duct, with excess keratin causing adherence of sloughed epithelial cells, blockage of the gland, cystic dilation and gland atrophy.11Alternatively, age-related increases in cellular debris in the central duct may lead to obstruction.12 It’s also possible that stress induced by contact lens wear or ocular surface drying can alter the stem cell proliferation and gene expression necessary for normal acinar function.11,13,14 Furthermore, with aging, there appears to be a decline in meibocyte differentiation and lipid synthesis, which can lead to meibomian gland dysfunction, gland atrophy, gland dropout and altered lipid synthesis. These studies suggest that hyperkeratinization may not play as great a role as previously thought in either the migration of the Marx line or in age-related MGD. If hyperkeratinization were the primary driver of MGD, then the position of the mucocutaneous junction would be expected to move posteriorly toward the gland orifice and not away from it.11 More definitive studies will be needed clarify and extend these observations.
Complicating Matters …
Lid health can be compromised and complicated by various conditions that can all ultimately exacerbate MGD. Seborrheic dermatitis, bacterial infections, dry eye and obstructions can all lead to a dysfunctional lid, lid margin and, consequently, tear film. However, like the proverbial chicken or the egg, often in these lid-related diseases it’s difficult to discern which pathology came first.
When MGD is suspected, it’s important to do a global assessment of the patient’s health, since non-ocular disorders such as rosacea are often associated with MGD. Similarly, the red, scaly patches of seborrheic dermatitis can be telling. This chronic inflammatory skin condition is found in areas with a dense distribution of sebaceous glands, and is more common during periods of increased sebum production.15
The bacteriological origin of sebaceous gland pathology also leads us to consider a bacterial origin of MGD. In lid skin, antimicrobial lipids are stored in epidermal granules and dispersed into the intercellular spaces of the epithelium, where they release antibacterial sapenic and lauric acids. These fatty acids act in synergy with tear lactoferrin and lysozyme. Many organisms are susceptible to these acids, such as S. aureus, S. pyogenes and S. epidermidis. A deficiency in the quality or quantity of meibomian secretions can compromise this innate defense mechanism, putting the lid margin at greater risk for microbial invasion and the development of MGD.6 Altered flora on the lids of patients with MGD may be an indication for the use of antimicrobial agents.
A number of ocular conditions are related to meibomian gland obstruction. Chalazia can cause abnormally thick meibomian gland secretions and increase the risk of meibomian gland obstruction. Foreign body giant cells are present in chalazia, yet they aren’t thought to be related to an infectious process. By contrast, a hordeolum may be present and is usually caused by infection. Most hordeola are external and result from obstruction and infection of an eyelash follicle and the adjacent glands of Zeis or Moll. An internal hordeolum, which is very rare, results from infection of a meibomian gland.
Dry Eye and MGD Therapies
The relationship between MGD and dry-eye disease is truly tenuous and may be viewed as overlapping circles in a Venn diagram. There’s a temptation to presume that MGD causes dry eye due to abnormal meibum secretion, leading to tear film instability and increased evaporation. However, it also may be true that evaporative dry eye precedes the development of MGD. Chronic lowering of the tear-film meniscus causes the delicate mucocutaneous shoreline to dry up, and hyperkeratosis, chronic inflammation, metaplasia and obstruction of the meibomian orifices can ensue. In this scenario, the development of MGD is a self-propelling process.
With the realization that the meibomian gland is a modified sebaceous gland, it follows that hormonal effects on gland function are likely to be significant.16 Sebaceous glands are relatively inactive until the teenage years, at which point they increase in size and secrete larger quantities of sebum. Sebum levels stay relatively constant until about 80 years of age in males and until menopause in females. Both the increase in gland activity accompanying puberty and the decrease observed later in life are attributed to changes in androgen production.
Also, the impact of hormone levels on meibomian glands are readily observed in male patients undergoing anti-androgen therapy for prostate disease. These men have increased rose bengal and fluorescein staining; abnormal meibum; decreased glyceride, wax ester and cholesterol ester concentrations; and an increased free cholesterol content.7 We now believe that acinar cells respond to androgens by activating expression of a host of genes, some of which encode enzymes involved in synthesis and secretion of meibum components.13
Managing lid-margin disease ultimately relies on the patient-doctor relationship. Evaluation should include a history of medication use, as a number of commonly used drugs can impact meibomian gland function in these patients.
If there are no overt signs of rosacea, dermatitis, hordeolum or chalazia, many clinical tools are available to evaluate MGD. Traditional examinations include slit-lamp biomicroscopy of the gland orifices, the Schirmer’s test, tear meniscus height measurement, fluorescein staining, meibographic assessment of gland structure by transillumination, and subjective questionnaires. More sophisticated assessments available to researchers include fluorophotometry; lipid analysis using mass spectroscopy or thin-layer chromatography;17-19 Ora’s Lid Imaging System;20 and the LipiView Ocular Surface Interferometer (TearScience, Morrisville, N.C.).21
Traditional treatments for MGD patients involve the use of warm compresses, lid scrubs, lid massage and gland expression with a cotton-tipped applicator. Although there is no one medication that works best for all patients, compounds that contain steroids seem to be the most effective in treating severe cases. One such therapy in development that was tested using Ora’s anatomically based scales is NCX 4251 (Nicox, SA, Sophia Antipolis, France), a novel formulation of fluticasone propionate that utilizes an applicator for topical delivery to the eyelid margin.
Topical tetracyclines such as doxycycline have proven beneficial due to antibacterial and anti-inflammatory effects. In a study conducted by Ora using the Controlled Adverse Environment model, which exacerbates the signs and symptoms of dry eye with a desiccating environment, ALTY-0501 (Alacrity Biosciences, Laguna Hills, Calif.), a formulation of doxycycline, protected MGD patients against environmentally induced keratitis, as shown by significant reductions in fluorescein staining scores compared to controls. (Shapiro A, et al. IOVS 2008;49:ARVO E-abstract 84)
A combination of agents may be required for inflammatory conditions in which superficial bacterial ocular infection exists. In a study sponsored by Alcon, TobraDex ST (tobramycin/dexamethasone, Alcon, Ft. Worth, Texas), which is a multiple-dose antibiotic and steroid combination, was shown to be faster than Azasite (azithromycin, Inspire Pharmaceuticals) in controlling the signs and symptoms of lid-margin disease.8LipiFlow (TearScience), a novel thermal pulsation system that applies heat and pressure to the eyelid tissue, has been shown to be effective in expressing meibomian glands.22
Meibomian gland intraductal probing in the form of the Maskin Meibomian Gland Intraductal Probe (Rhein Medical, St. Petersburg, Fla.) is a procedure that physically removes material obstructing the gland ducts using a 1- or 2-mm probe. Probing is indicated in patients who complain of lid tenderness or who have symptoms such as burning and stinging and manual lid compression produces little to no sebum.
Meibomian gland secretion plays a crucial role in ocular surface health. Lipids secreted by meibomian glands have an essential role in reducing evaporation from the ocular surface, lowering the surface tension of tears, preventing tear spillover and preventing damage to the skin of the lid margin. Diagnostic accuracy—putting a name to the diagnosis—is the first step in a successful therapeutic strategy. No matter what name we give them, though, related tear-film disorders including blepharitis, meibomian gland dysfunction and meibomitis all have the potential to impact the health of the ocular surface.
Find out more about our eye tissue here.
